Project Management + Quality Management Software (SaaS)
Introduction:
Being compliant with FDA 510(k) and ISO 13485 is a significant challenge for medical device producers. Each step of product development necessitates extensive paperwork, increasing the likelihood of errors and requiring significant preparation time. To mitigate these challenges, the client sought a unique application capable of ensuring compliance efficiently.
Challenges:
-
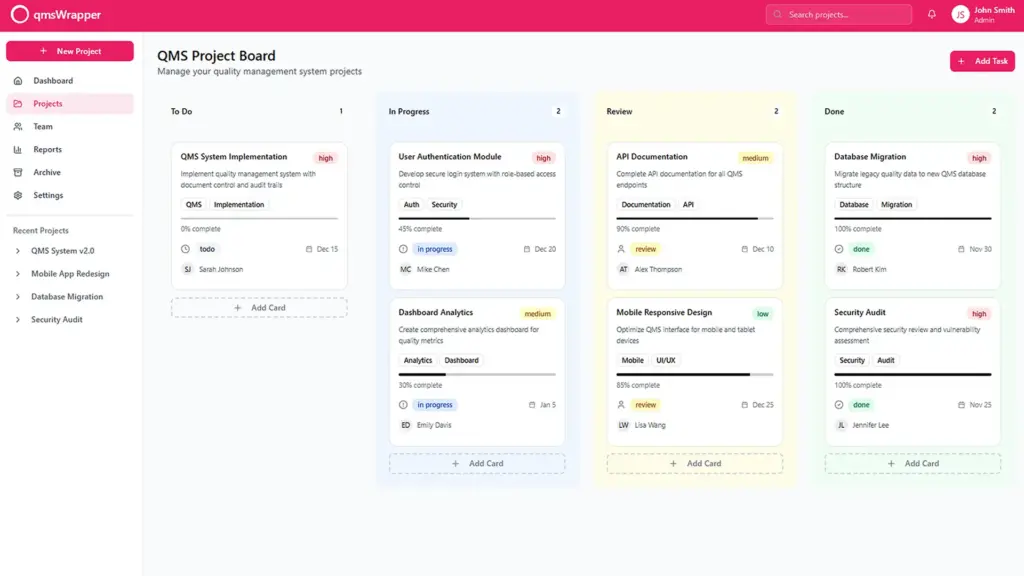
Avoid paperwork in the production process of medical devices, streamline technical file management.
-
Fixing issues with project organization occurs across all stages of medical device production.
-
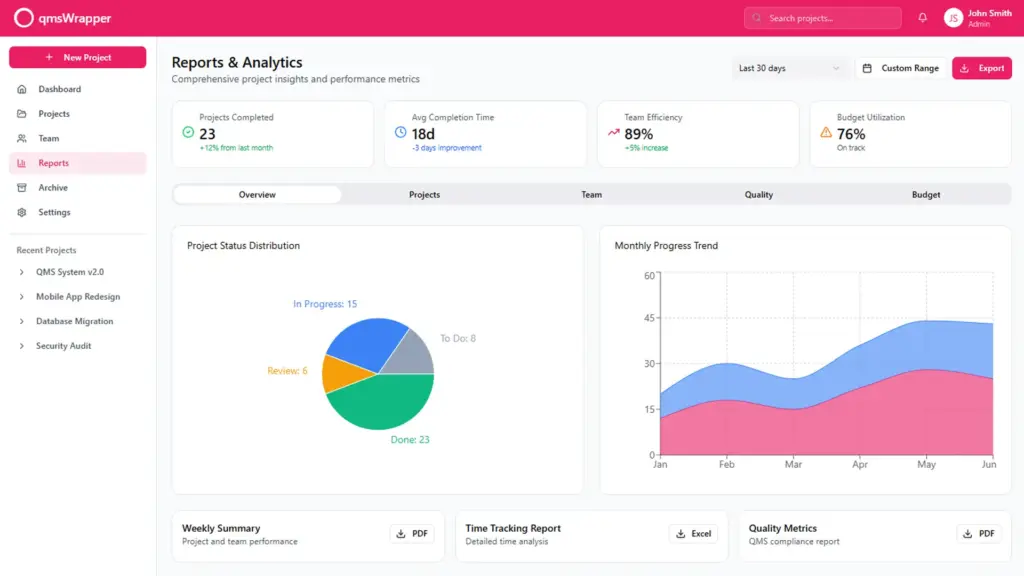
To gain control over risk management, involve the team in all production stages.
-
Ensuring alignment with EU regulations is crucial for medical device producers operating in the EU.
Solutions:
-
UX flow was adjusted the way to follow risk management regulations in order to be compliant with FDA 510(k).
-
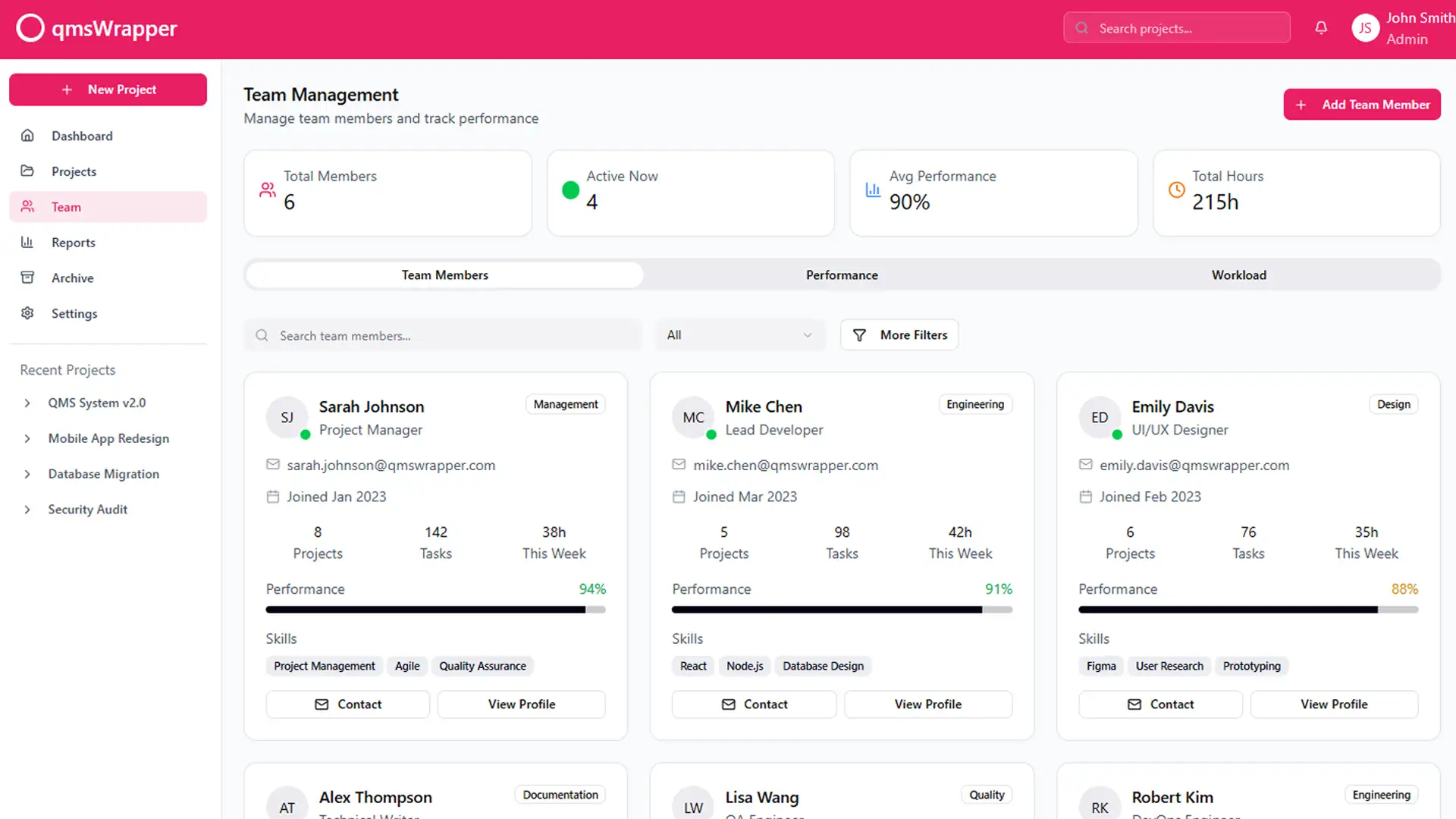
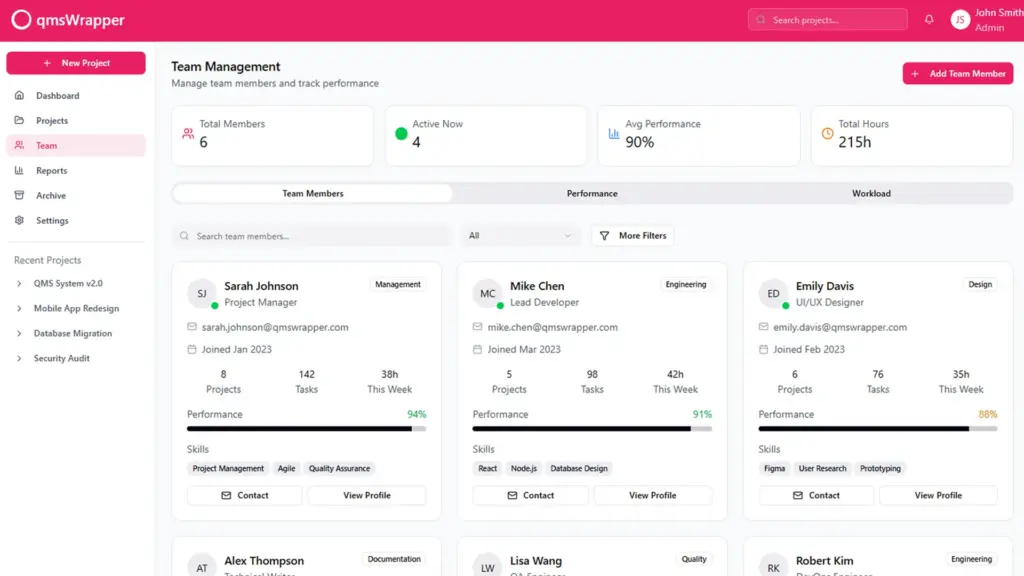
The application needs modification to offer a step-by-step sequence of tasks with sufficient explanations and cautions to assist QMS Managers in performing their duties accurately. This involves creating a comprehensive loop of follow-up and coordination for each quality objective, task, and issue by guiding projects through QMS processes.
-
Managing through quality entails team members completing their tasks, which in turn triggers QMS events automatically reported to the QMS system. This allows managers to oversee progress, not only in project management but also in any QMS management aspect, without imposing on routine operations. This approach aims to reduce errors, prevent forgotten paperwork, and ensure no missed QMS reports through workflow processes that leave no room for guessing what comes next. Steps are defined, monitored, and managed automatically by management.
-
The Risk Module encourages focusing on the relationship between requirements and design, with an emphasis on risk-based design. It offers flexibility, making it suitable and proportionate to the complexity and type of organizations. Designed to be accessible at any time, users need not reinvent the wheel with each use. All necessary information, such as hazardous situations during the risk identification phase or hazard categories, is graphically mapped out on a single interface for a clear overview. Additionally, it is adjusted to comply with all USA and EU regulations regarding medical device production.
Results:
-
More than +500 companies trusted qmsWrapper with their QMS.


